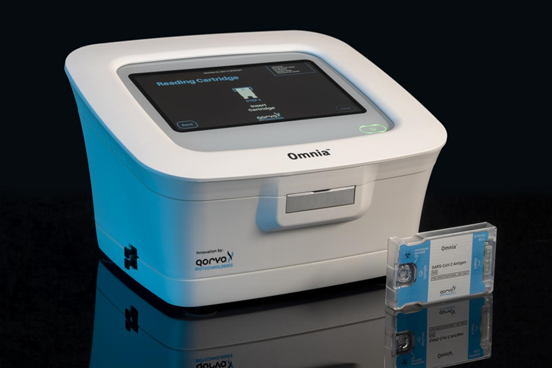
Qorvo®, Inc, a leading provider of RF solutions for mobile applications, infrastructure and aerospace and defense applications, announced that the U.S. Food and Drug Administration (FDA) has issued an Emergency Response to Qorvo’s Omnia™ SARS-CoV-2 Antigen Test Use Authorization (EUA) for use in Point-of-Care (POC) environments.
The test is authorized for qualitative testing to determine the presence of SARS-CoV-2 (COVID-19) nucleocapsid virus antigens in nasal swab specimens from patients with suspected COVID-19 within six days of symptom onset. In addition, the test is authorized to be used in at-risk groups temporarily without suspected symptoms of COVID-19 or other epidemiological manifestations, with the rule being to test twice within three days, with an interval between tests of no less than 24 hours and no more than 48 hours. The FDA has previously issued an emergency use authorization for the test, approving its use in medium- and high-complexity settings such as laboratories. This Emergency Use Authorization expands Qorvo's market beyond the laboratory to include physician offices, emergency centers, retail pharmacies, employee health testing, and any other location where Clinical Laboratory Improvement Amendments (CLIA)-exempt testing is available.
Erik Allen, Qorvo's new vice president and president of Qorvo Biotechnologies, said, "As COVID-19 testing becomes more common and viral loads of Omicron variants decrease, point-of-care settings require a high-quality rapid testing infrastructure. Qorvo The Omnia platform offers a unique combination of performance, automated workflow and scalability to efficiently meet field inspection needs."
The Qorvo Omnia platform utilizes innovative diagnostic technology through high-frequency bulk acoustic wave (BAW) sensors to rapidly produce highly sensitive and specific COVID-19 test results on an easy-to-use stand-alone platform. BAW sensor technology achieves low limit of detection (LOD) levels that approximate molecular detection capabilities. As viral loads decline, especially with the emergence of Omicron variants and subtypes, low viral loads in patient samples pose challenges to existing over-the-counter (OTC) lateral flow assays . The Qorvo Omnia platform has the technical advantages to continuously and accurately detect the relevant antigens of COVID-19 during the peak period of Omnia.
The Qorvo Omnia platform excels at comparing the limit of detection (LOD) of PCR methods. The Omnia platform demonstrated 85% sensitivity (PPA) during clinical studies, supporting emergency use authorization of the device for point-of-care use; this project is funded by a National Institutes of Health (NIH) grant and is officially launching in the second half of 2021. NIH-funded independent research during the widespread dissemination of the Omicron variant in early 2022. In this study, Qorvo performed well when samples met or exceeded the detection limit of the comparative PCR method, with a sensitivity of 86%. Specificity was 100% in all relevant studies.
Peter Matos, president and CEO of medical consulting firm Traeokos, said: "Today's testing needs are changing with Omicron variants, and Qorvo's antigen tests ensure customers the performance they need in a variety of testing scenarios. Level."